Validation of Results: Planning InteropEHRate clinical pilots
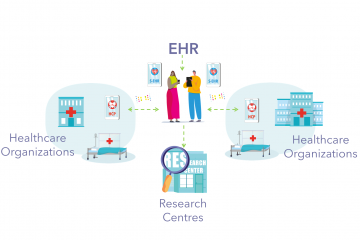
Clinical use of InteropEHRate solutions for the three scenarios – healthcare access, emergencies and research – will be tested in four European hospitals: Fondazione Toscana Gabriele Monasterio (Pisa, Italy); Centre Hospitalier Universitaire de Liège (Belgium); Hygeia – Diagnostic and Therapeutic Center of Athens (Greece); and “Bagdasar-Arseni” Clinical Emergency Hospital of Bucharest (Romania).
More than 50 patients will participate in the pilots to be conducted in early 2022. The healthcare and emergency access scenarios will involve 3 patients per centre while the research scenario will involve 10 patients per centre. Around 25 patients will participate in the study design process.
Cooperation with healthcare professional representation organisations (the European Federation of Nurses and the College of Physicians in the Athens Region), technology companies (Engineering – Ingegneria Informatica S.p.A., UBITECH Limited, A7 Software and SIVECO Romania S.A.) and two universities (University of Trento and University of Vienna) will improve the implementation process.
Before the pilots, it is required a validation plan and ethical approval in each site which is expected by the end of 2020.
Three main activities compose the validation of InteropEHRate results:
- Development of the clinical research protocol that will involve the use of data coming from the two first scenarios and data entered by patients.
- Platform assessment protocol by citizens, HCP and researchers: relies on the principles of human-machine ergonomics, resilience to human errors, data availability, data readiness, user expectation upon defined functionalities, level of awareness and confidence of device/tools use, and perceived level of trustfulness.
- Privacy impact assessment: including the evaluation of the InteropEHRate platform.
Ethical committee approvals are based on detailed clinical protocols of the experimental use of the platform collecting a specific dataset of patient’s information for each scenario. Initial submission is foreseen for January 2021.
As main result, a document package will be ready for submission to reference Ethical Committees by the end of 2020. It will include the full clinical research protocol, the validation documents for healthcare professionals, researchers and patients and the DPIA.