In a nutshell
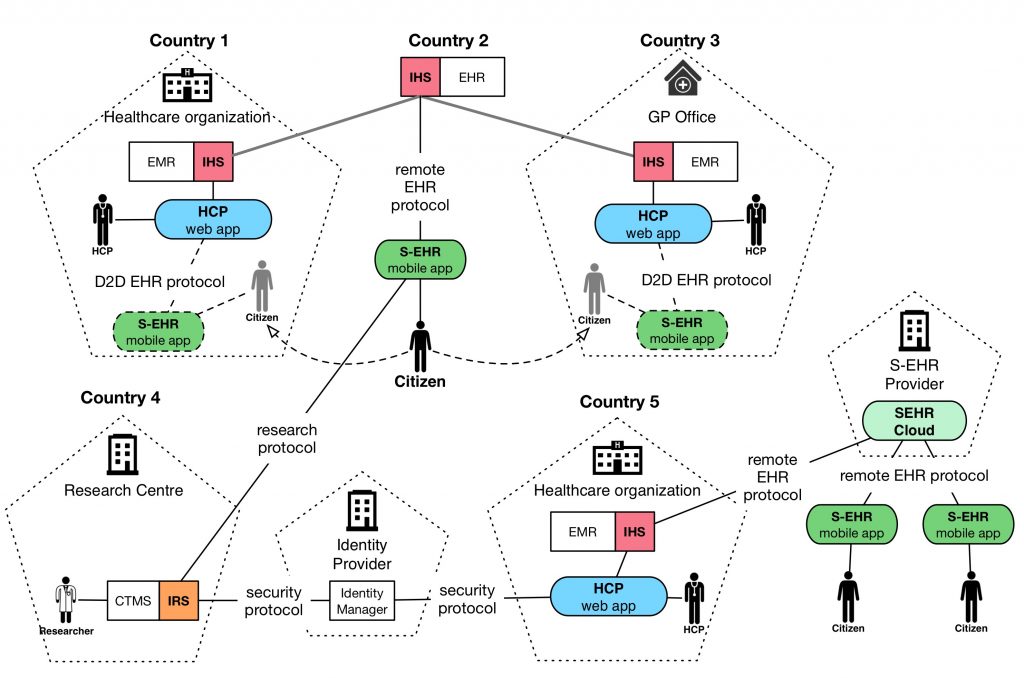
InteropEHRate enables patients to be in full control of the use of their health data. Through Smart EHR (S-EHR), InteropEHRate leverages a set of new communication protocols for secure and cross-border exchange of health data. An S-EHR (also called S-EHR App) may securely store the health history of a person on his or her mobile device. S-EHRs can communicate with applications of health care providers and researchers of different countries via highly secure channels of two kinds: local (based on a Device-to-Device protocol that does not use the Internet), remote (using Internet-based protocols).
At people’s choice, an S-EHR App may be complemented by the usage of an S-EHR Cloud service for preserving personal health data also on a cloud repository managed directly by the citizen. Health data are sent to the S-EHR Cloud in an encrypted format so that the health information is hidden to the Cloud provider.
An S-EHR supports the following communication protocols:
- D2D (Device to Device) protocol: Exchange of data between patients and healthcare organizations without an internet connection.
- R2D (Remote to Device) protocols: Three protocols for remote access: (1) to EHRs for citizens and to optional S-EHR Cloud for (2) citizens and (3) healthcare providers.
- RDS (Research Data Sharing) protocol: Sharing of health data with research centres without cloud storage.
Healthcare visit abroad scenario
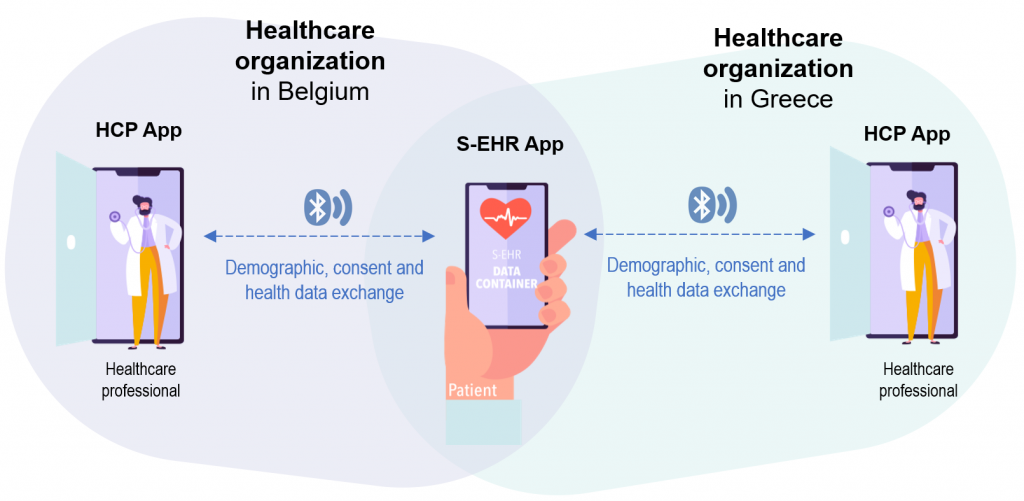
This scenario shows how citizens and healthcare providers may exchange clinical data through their devices without the use of the internet and cloud storage.
A Belgian patient suffers from chronic ischemic heart failure and atrial fibrillation. The patient is regularly followed-up at the outpatient clinic of a tertiary centre where he undergoes twice a year EKG and blood tests, and yearly, echocardiogram, cardiopulmonary exercise testing, device control and 24-hour Holter monitoring, together with cardiological consultation.
The patient moves to Greece for a 2-year stay during which progressively complains of mild lower limbs oedema, dyspnoea, and reduction in exercise tolerance.
Demographic, consent, and previous history of the patient were already loaded on the S-EHR App. During her stay in Greece, the patient seeks medical care. At the healthcare visit and using the S-EHR App, the patient authorises the healthcare professional to access elements of her health data such as allergies or adverse drug reactions. The clinician accesses this shared data from an HCP App able to make use of the D2D protocol. Treatment is established on these grounds and any prescription is transferred to the S-EHR of the patient’s mobile. Once the patient has left, the doctor has no access to further, additional information from the S-EHR. At return in Belgium, the patient will be able to similarly exchange the newly collected health data with other HCPs using the D2D protocol.
Emergency access scenario
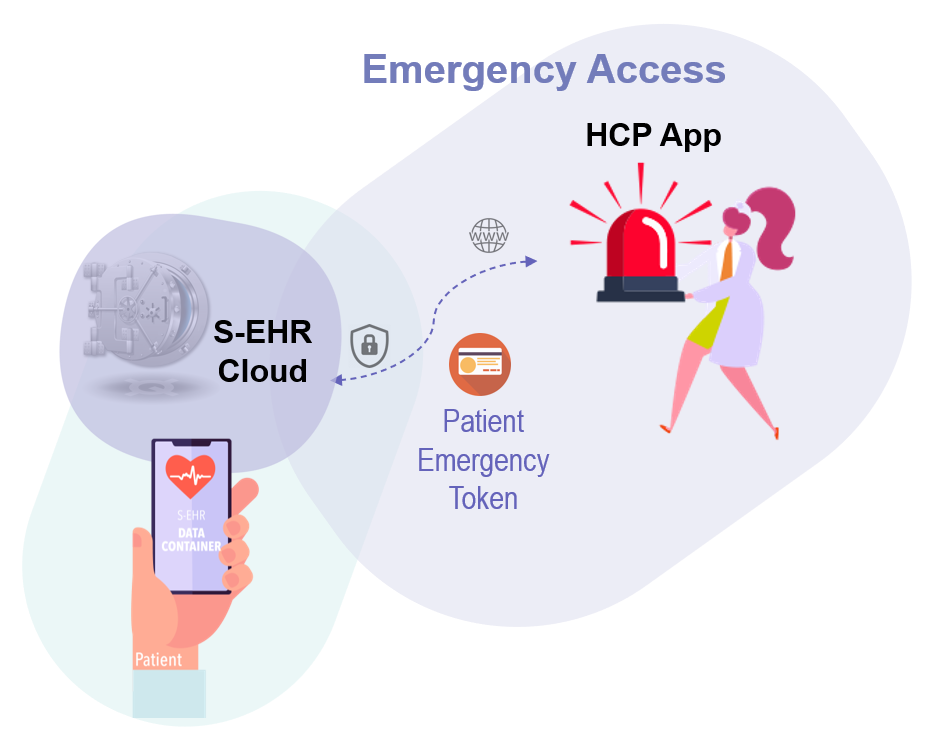
This scenario shows how HCPs may access and contribute to Patients’ health data when an S-EHR App is not available or when the Patient cannot use it, in particular in an emergency situation.
A 56-year-old male patient is abroad and complains nausea, vomiting and mild abdominal pain. He is transferred by an ambulance to a local emergency department. The condition deteriorates, the patient is in a numbed state and requires prompt treatment.
The patient previously enabled the S-EHR Cloud and brings with him a personal identity token for emergency use. Emergency care providers use an HCP App that supports the R2D protocol for healthcare. Following the protocol, the application verifies their identity and professional role using the national identity provider. Using the patient emergency token and thanks to their authenticated identity and role, the HCPs are granted access to the emergency dataset stored in the S-EHR Cloud of the patient. The access is traced using “emergency mode” and recording the credentials of treating healthcare professionals. Patient’s health data are retrieved from the S-EHR Cloud, translated into the local language and terminology to support a risk-free diagnosis and treatment of the patient. At patient discharge, the S-EHR Cloud is updated by the HCP with a Discharge Report containing the cause of admission, discharge diagnostic assessment, prescriptions, visits and recommendations, therapy and prescriptions. The new health data will be available on the S-EHR App thanks to the R2D protocol for citizens.
Research data sharing scenario
This scenario shows how citizens can share health data with research centres without cloud storage enabling decentralised clinical trials.
The research centre of the Regional University Hospital conducts a research study about the incidence and risk factors for a medical condition in the general community. The research protocol requires the prospective collection of anonymised health data for 2 years after the patient enrolment and a 5-year retrospective evaluation of the patient’s data.
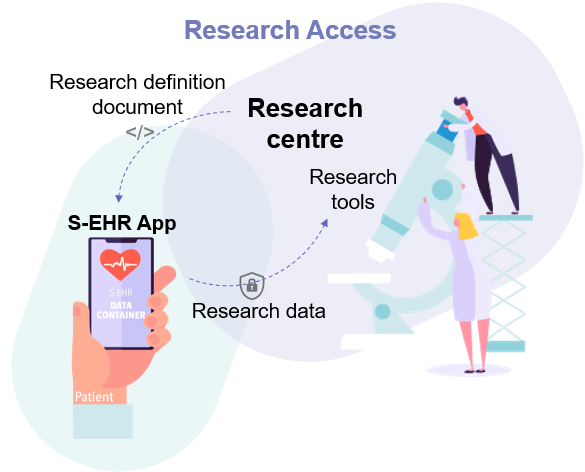
Mrs Eve, a patient with chronic hypertension, has learned through her S-EHR App, that the Regional University Hospital is conducting a study called “Side effects from hypertensive medication study” using the InteropEHRate format.
Being altruist by nature she finds out on the S-EHR App more information about the research and in particular the kind of data requested for the research. Eve accepts the invitation to participate in the study and signs the consent form with her smartphone. The research protocol requires sharing the health data of her previous 5 years and for the next 2 years, restricting their use only to that specific research protocol. Eve is asked to fill a questionnaire on self-reported side effects from antihypertensive medications.
A few months later Eve complained of nausea probably related to the assumption of the antihypertensive medications. Eva opens her S-EHR app and accesses the questionnaire of the research protocol, compiling the questionnaire with the symptom. The questionnaire is sent to the University Hospital conducting the research. Eve can withdraw her participation in the research at any time. In the case of withdrawing, the event is anonymously notified to the Research Centre of the University Hospital.
Infrastructure Building Blocks: software components and interfaces to be specified and/or prototyped by the project
The project will release an open specification for allowing different S-EHRs, of citizens, and different applications, of researchers and HCPs of different countries, developed by different vendors, to securely exchange health data using the InteropEHRate protocols. To facilitate the creation of a new ecosystem of applications based on this open specification, InteropEHRate will also release a reference implementation of the following key elements:
- S-EHR mobile app: the prototype of a S-EHR, i.e. of a personal mobile app able to store in a secure way any health data about a single citizen, produced by the citizen itself or by the HCPs. The S-EHR mobile app will be able to exchange health data with HCPs and researchers of different countries using the InteropEHRate protocols.
- S-EHR cloud: the prototype of a service, managed directly be the citizen, able to store on the cloud the personal health data of the citizen, collected by the S-EHR mobile app. A citizen may choose to use the S-EHR mobile app without using the corresponding S-EHR cloud.
- InteropEHRate Health Services (IHS): a set of service components, reusable by any healthcare organization, offering the implementation of the InteropEHRate protocols for the exchange of health data between S-EHRs and HCP Apps, i.e. between citizens and HCPs. The IHS will exploit existing infrastructures for cross border identification of citizens and will assure the respect of strong security requirements.
- HCP Web App example of a typical application exploiting the IHS and used by Health Care Professionals to read from and write on the S-EHR of their patients, with their consent, any relevant health data.
- InteropEHRate Research Services (IRS): a set of service components, reusable by any research centre, offering the implementation of the InteropEHRate protocols, for demanding to the citizens and receiving from their S-EHRs, health data donated for research purposes.